

The CordenPharma Solid State Centre of Excellence, located at our Liestal, Switzerland site, provides extensive solid-state API development support services tailored to your project needs. Our dedicated team of crystallization process and solid-state form experts, combined with the latest technology and equipment, allows for a bespoke offering that includes salt and co-crystal programs, polymorph investigations, crystallization process development, and scale-up investigations.
The importance of crystallization & solid-state forms
Crystallization is the predominant separation and purification process used in the production of a wide range of solid materials. Together with isolation (washing and filtration) and drying, it is the last operational step in the process of API development where many physical properties of the final solid material can be massively influenced in a controlled way. Such properties like purity, or particle size distribution, are generally considered critical quality attributes, and hence it is of utmost importance to develop processes that consistently deliver the same quality. Generating process understanding using PAT analytics allows the development of robust, safe, scalable, and economic processes.
Crystallization of intermediates is often an underestimated asset. Due to the purification effect of crystallization, this process is very useful to better control carry-over of unwanted impurities along the synthesis route, which is particularly of interest for potentially genotoxic impurities. In addition, such novel crystalline key intermediates, which are also used as regulatory starting materials, can lead to additional patent protection.
While the goal of the crystallization process consists of making a target bulk solid, the target solid-state form needs to be defined first. Since different solid-state forms have different physico-chemical properties, the crystallization process therefore also provides a great opportunity to target the optimal solid-state form for the intended use. In the context of low solubility compounds and oral delivery of solid drug products, the pH dependent solubility profile of such solid-state forms directly influences properties such as fraction absorbed and bioavailability, chemical and physical stability, melting point, and processability, just to name a few. Our carefully designed and executed salt, co-crystal, and polymorphism investigations lead to the target solid state form, becoming an integral part of any biopharmaceutic consideration for drug product development. Novel solid-state forms are often a way to generate valuable additional intellectual property.
Below, we present some of our latest case studies which highlight the great value of a rational approach to crystallization, and its use in API process development, here applied to complex lipid type molecules.
Improving purity by adding control to the final crystallization step
Complex lipids are typically molecules that have large flexible chains with only a limited number of functional groups, and for complex modalities, are branched at multiple sites. Due to their complex nature and multistep synthesis, similar by-products are easily carried over, making these molecules difficult to crystallize with a good final purity of >95%.
The crystallization process carried out in our Solid State Centre of Excellence showed the improvement of a complex lipid within a short time to ensure the purity consistently met the target specifications of >95%, and was free of agglomerates, while the initial process consisted of an unseeded cooling crystallization that resulted in only 87% purity, and appearance of agglomerates.
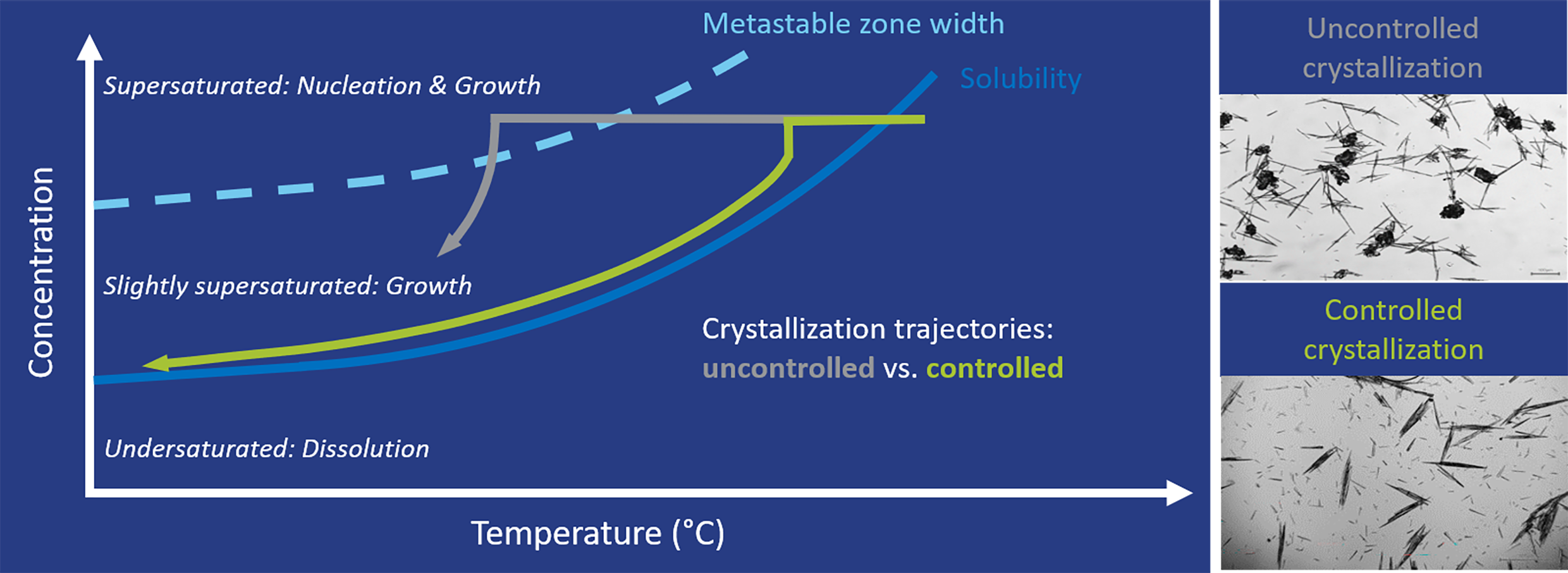
[CordenPharma Figure 1] Crystallization design and trajectory (green) based on solubility and metastable zone width data for a controlled seeded cooling crystallization to ensure a robust and repeatable process that provides the desired crystal form, size, purity, and yield.
The first step consisted of estimating the solubility at a very small scale in solvents to identify suitable systems for crystallization optimization. Using small-scale automated lab reactors equipped with turbidity sensors, the temperature dependent solubility curves and a first impression of the Metastable Zone Width (MSZW, Figure 1) were generated. This data was used to calculate and design a theoretical seeded cooling crystallization process, which included the starting concentration, supersaturation ratio, seeding region and end temperature.
The crystallization was then experimentally tested at small-scale. Cooling rates were optimized during scale-up, now using automated lab reactors equipped with turbidity and imaging sensors (Figure 2). The addition of a seeding step, combined with a slower cooling rate to allow sufficient crystal growth time, and lower end temperature, resulted in an increased purity of 96%, and a good yield. In addition, the undesirable agglomerate formation was eliminated. This ensured the process would reliably and repeatably produce the desired form with controlled critical quality attributes.

[CordenPharma Figure 2] In-situ process analytical technology enables recording of real time micrographs which allows for tracking of the controlled crystallization process achieved by seeding and Ostwald ripening.
Salt formation to crystallize an oily intermediate and isolate a pure, stable, and well-behaved powder
A key intermediate of a complex lipid resulted in an undesirable oil that was a free base, with a low purity of 93%. The CordenPharma team performed a salt screen utilizing very small-scale, automated and parallel lab reactors. Three crystalline salt forms of the intermediate were discovered and produced in sufficient quantity for a solid-state characterization and stability evaluation. The optimal salt that exhibited the most favorable properties was chosen for scale-up investigations and further development. This allowed the development team to progress the project rapidly with an intermediate that can be isolated, was stable, could be easily stored, and that exhibited a higher purity of 96%, which was of great value for the subsequent synthesis steps.
In the next Corden Connect edition we will discuss our solid-state form approach and its relevance in the biopharmaceutic decision tree for the development of high-performance Oral Solid Dosage (OSD) forms of low soluble compounds.
Want to find out more?
Get in touch with our team of experts to explore bespoke end-to-end CDMO support of your complete drug lifecycle
at any scale.




