

[Photo by CordenPharma] Air jet mill development at the new Drug Product Innovation Centre of Excellence in CordenPharma Plankstadt (DE).
In April 2023, CordenPharma announced the establishment of the Drug Product Innovation Centre of Excellence (DPICoE) at our CordenPharma Plankstadt site near Heidelberg, Germany, to provide additional capabilities for developing new innovative APIs. The benefits of the DPICoE include the ability to formulate APIs into Oral Solid Dosage (OSD) drug products which have challenging properties, such as peptides and small molecules with limited solubility, permeability, very low drug load, high potency and limited stability. In addition, together with our Solid State Centre of Excellence in CordenPharma Liestal, Switzerland, we now provide a fully-integrated API and Drug Product development approach by combining the optimal physical API form required with a drug product formulation that allows for sufficient absorption / solubility, stability, and processability. In this way, we run a comprehensive screening using a very small amount of API.
Already equipped with conventional small scale OSD development processes including blending, granulation, compression, coating, and a tablet compression simulator, the Drug Product Innovation Centre of Excellence now includes a variety of enabling technologies including spray drying, hot melt extrusion, nanomilling, and micronization. These technologies can be applied to formulations that need particle size reduction, an amorphous solid dispersion, or a lipid-based drug delivery system. Because API supply in preclinical and early phase is normally very limited, this added ability to initiate development activities with only a few grams of API will be of great benefit to a broad range of customers from emerging biotechs to mid and larger pharma programs. In addition, the DPICoE is able to handle HPAPIs without any restrictions, is capable of handling multiple projects in parallel, and has the flexibility to start projects immediately.
An integrated development approach in early phases is important in order to move the right formulation through the clinic and avoid unfavorable surprises in the late phase. In addition, existing formulations currently in clinical development or already marketed commercial products that do not have sufficient performance with regards to manufacturability, stability, or bio-performance can also be improved by API modification, formulation optimization, and/or process optimization without any technology limitations. 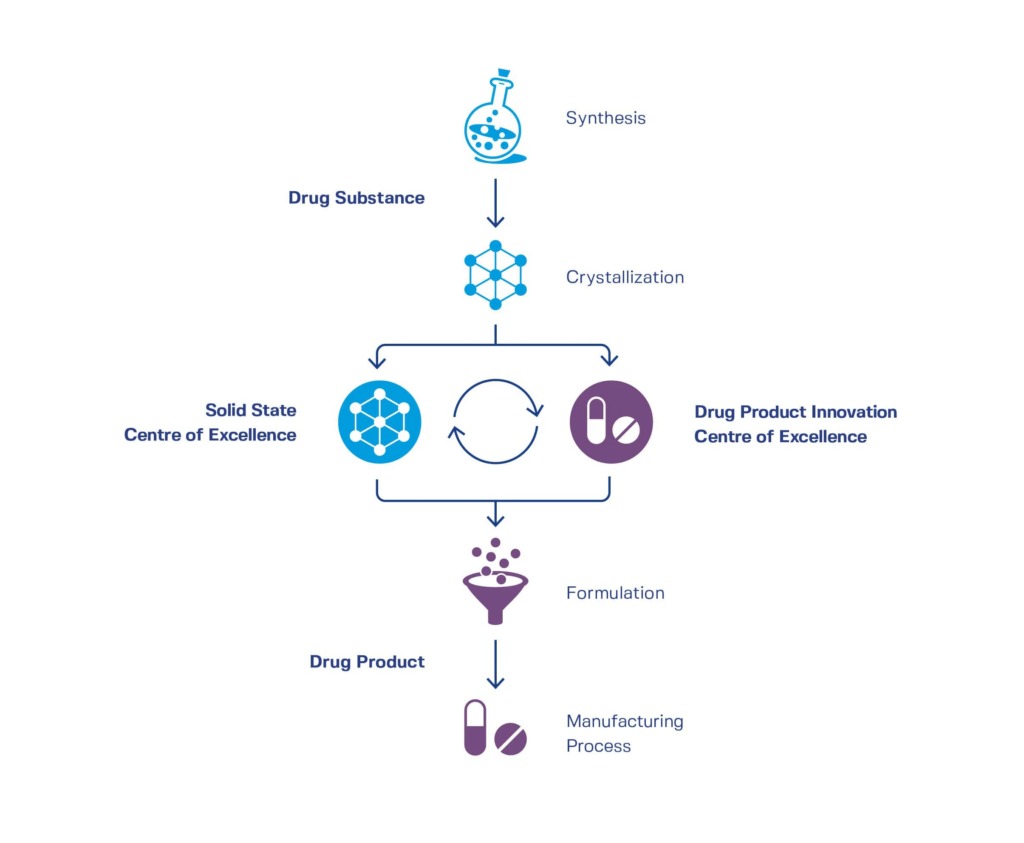
[Graphic by CordenPharma] Integrated drug substance and drug product development.
Getting a product into First-in-Human (FIH) clinical Phase I studies in a flexible, fast, and efficient manner is important. For this reason, the DPICoE was designed to offer customers an initial developability assessment that includes API profiling and technology selection (conventional, bioavailability enhancing), followed by a screening study (excipients, polymers, and/or technologies), phase-appropriate formulation development, and prototype preparation. Utilizing the Accelerated Stability Assessment Program (ASAP) for stability, CordenPharma can identify the best prototype to manufacture and advance your project into FIH studies quickly.
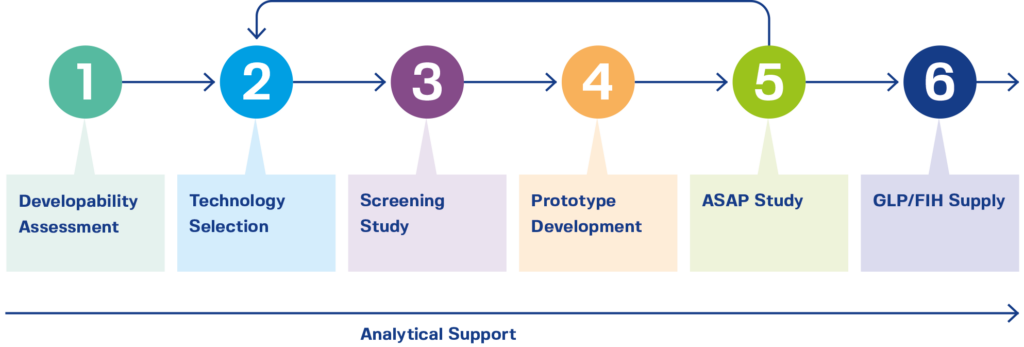
[Graphic by CordenPharma] Capabilities for developing First-in-Human clinical materials.
Depending on the API’s properties, the DPICoE team applies the most appropriate solubility enhancement technologies. For example, amorphous solid dispersions can be developed using spray drying or hot melt extrusion and particle size can be reduced to 1-5 µm by an air jet mill, or even smaller with nanomilling (wet ball milling). In this case, since the API nanoparticles have a much faster dissolution rate due to the tremendous increase in surface area by keeping the API crystalline, the wet suspension can then be used to manufacture the final drug product with conventional equipment.
For peptides or large molecules that exhibit limited bioavailability caused by poor permeability through the intestinal barrier, lipid-based formulations or permeation-enhancer-based formulation technologies are required. To address these challenging APIs, we apply innovative formulation approaches to provide prototypes for animal in vivo investigations. All formulations can be manufactured as a solid form and allow a larger production scale.
As timelines become more aggressive for development projects, stability studies of prototype formulations become more and more critical. Although stability studies are required to assess new or improved formulations, they are also time consuming and dictate timelines for clinical studies and regulatory submissions. To address this challenge, we utilize the ASAP program for predicting chemical stability and shelf-life, and then identify the best prototype to manufacture and advance into cGMP manufacturing for FIH studies in weeks versus months. Additionally, the ASAP study de-risks decisions, reduces costs, and supports dosage form and packaging selections.
For formulations that contain an API with limited stability or incompatibility in the current formulation, or have existing manufacturing challenges, CordenPharma’s new Drug Product Innovation Centre of Excellence offers a broad range of small-scale process equipment for conventional and enabling technologies, where even API physical properties can be improved to tune formulation and process performance.

[Photo by CordenPharma] CordenPharma’s Drug Product Innovation Centre of Excellence Team in Plankstadt (DE). From left to right: Giorgio Marino, Stefanie Avril, Oliver Schinzinger and Alexander Denninger.
Want to find out more?
Get in touch with our team of experts to explore bespoke end-to-end CDMO support of your complete drug lifecycle
at any scale.


