[Photo by CordenPharma] Freeze drying isolation of APIs in the containment area of CordenPharma Frankfurt.
The Frankfurt site will add 1000 m2 of manufacturing space, including two fully equipped lines comprised of a Solid Phase Peptide Synthesizer (SPPS), High Pressure Liquid Chromatography (HPLC), Liquid Phase (LP), isolation equipment and quality control laboratories including In Process Control (IPC), starting material, API batch release, and GMP stabilities. The GMP manufacturing area will be designed to produce peptide APIs from gram to kilogram range for clinical phase 1 and 2 requirements. As the project progresses along the customer lifecycle, the new state-of-the-art technologies will enable a smooth and seamless transfer to the late phase and commercial manufacturing site CordenPharma Colorado (Boulder, US).
Moreover, the GMP expansion supports the launch of an integrated service offering between CordenPharma Frankfurt for Peptide Drug Substance and CordenPharma Caponago (IT) for Injectable Drug Products to deliver fully customizable technical, manufacturing, and regulatory support that is specifically targeted to enable efficient IND / IMPD filings, with all the necessary materials needed to initiate customers’ First In-Human (FIH) clinical trials.
Through our integrated peptide-injectable offer, customers will benefit from:
- One CDMO relationship with a single contract, including quality agreement & project management
- API route selection, salt & solubility studies, API characterization, reference standard qualification
- Formulation development, analytical method development & validation
- Stability studies for development, toxicology & GMP batches
- Technical writing for the IND / IMPD submission
CordenPharma delivers a unique integrated peptide offering tailored for to Biotech innovators targeting IND / IMPD filings.
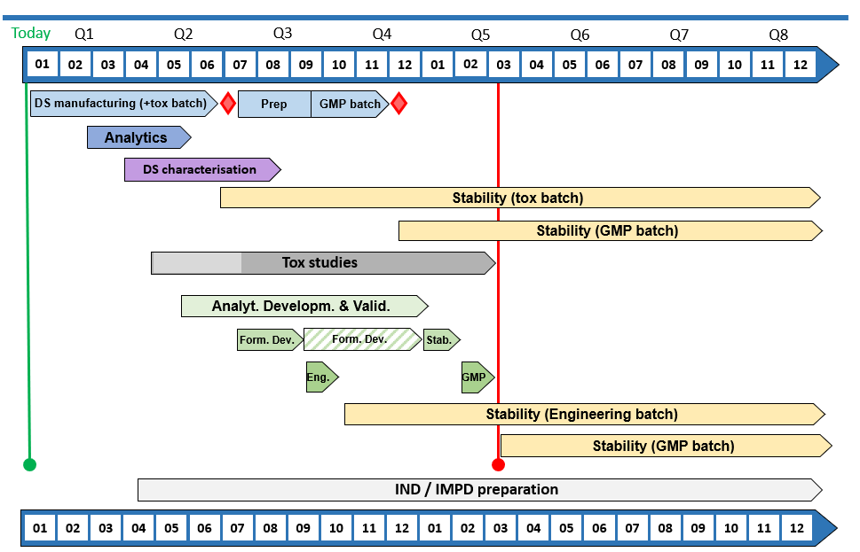
CordenPharma example timeline & scope for an integrated peptide-injectable project delivering GMP Vials within 14 months.
Our bespoke offer is tailored for each individual customer and will enable pharma and biotech innovators to move their complex modalities quickly to tox batch and FIH clinical trials, while actively managing business requirements and balancing timelines and budget, without losing track of important project milestones.
Please contact us for more information on our peptide-injectable offering.
Want to find out more?
Get in touch with our team of experts to explore bespoke end-to-end CDMO support of your complete drug lifecycle
at any scale.