
CordenPharma Invests in Innovative Purification Technologies: A Greener Approach to Highly Pure Complex Lipids
2 December 2021 – Luxembourg >
Since the beginning of the pandemic, CordenPharma has been continuously investing in their facilities in Switzerland, France, Italy and the USA to contribute to the lipid supply chain needed for the increasing demand of mRNA COVID-19 vaccines. As part of this long-term strategy, CordenPharma is pleased to announce the investment in an expansion of its specialty lipids production at CordenPharma Chenôve (FR) using Supercritical Fluid Chromatography (SFC) technology for compound separation, which is an efficient and cost-effective process for purifying lipids and pharmaceutical drug substances.
Because SFC is an eco-friendly and sustainable technique using reclaimed CO2 coupled with online carbon dioxide recycling, the resulting increase in lipid production will be a greener approach to manufacturing highly pure complex lipids, which are essential for not only mRNA-based COVID-19 vaccines, but also for newly developed cell and gene therapies, SiRNA and miRNA programs. Commercial lipid quantities using the new technology will be produced at CordenPharma Chenôve as early as the first half of 2022.
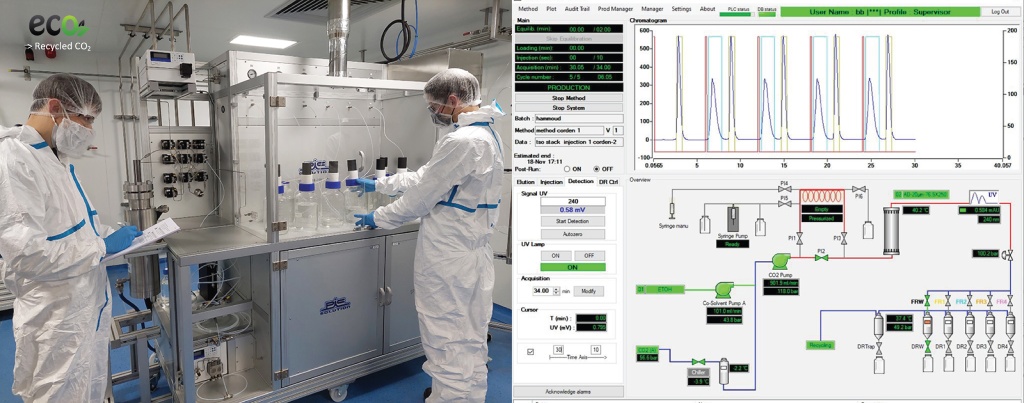
“Manufacturing of highly pure lipids is a very unique capability involving complex production processes, and CordenPharma is differentiating itself with this innovative SFC purification investment as a reliable partner for the pharmaceutical industry, far beyond the demands initially brought on by COVID-19. This technology was selected for its versatility and high throughput, and because it supports our green efforts to significantly reduce our carbon footprint,” comments Dr. Matthieu Giraud, Director, Global Peptides, Lipids & Carbohydrates Platforms.
CordenPharma supports mRNA vaccine development by supplying four classes of high purity lipids generally required to formulate Lipid Nanoparticles (LNPs):
- Cationic lipids that encapsulate the negatively charged mRNA;
- PEGylated lipids which help form a protective hydrophilic layer that sterically stabilises the LNP;
- Distearoylphosphatidylcholine (DSPC) phospholipids that provide a stable bilayer-forming structure; and
- BotaniChol®, CordenPharma’s non-animal origin cholesterol that stabilizes the LNP structure and facilitates endosome escape.
CordenPharma has been a Contract Development & Manufacturing Organization (CDMO) leader in advanced drug delivery for many decades. Not only do they provide expertise in lipid excipient manufacturing, but they also support pharmaceutical companies worldwide with the development and production of complex parenteral drug products, particularly those requiring formulation technologies such as Lipid Nanoparticles, at their CordenPharma Caponago site in Italy.
Want to find out more?
Get in touch with our team of experts to explore bespoke end-to-end CDMO support of your complete drug lifecycle
at any scale.

